To insert a bio-deposit citation, position where you want it to appear and click on Insert and then Bio-Deposit.
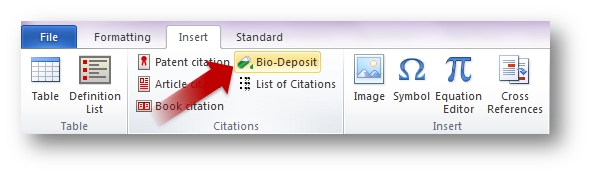
Enter the information relating to the biological material. To
delete the citation simply go to the end of it and use backspace.
Video: 3.3.4 Insert/change bio-deposits
Notes:
When providing biological material information, Rules 31 and 33 of the European Patent Convention apply:
Rule 31:
"(1) If an invention involves the use of or concerns biological material which is not available to the public and which cannot be described in the European patent application in such a manner as to enable the invention to be carried out by a person skilled in the art, the invention shall only be regarded as being disclosed as prescribed in Article 83 if:
- a sample of the biological material has been deposited with a recognised depositary institution on the same terms as those laid down in the Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure of 28 April 1977 not later than the date of filing of the application;
- the application as filed gives such relevant information as is available to the applicant on the characteristics of the biological material;
- the depositary institution and the accession number of the deposited biological material are stated in the application, and
- where the biological material has been deposited by a person other than the applicant, the name and address of the depositor are stated in the application and a document is submitted to the European Patent Office providing evidence that the depositor has authorised the applicant to refer to the deposited biological material in the application and has given his unreserved and irrevocable consent to the deposited material being made available to the public in accordance with Rule 33.
(2) The information referred to in paragraph 1(c) and (d) may be submitted
-
within sixteen months after the date of filing of the application or, if priority has been claimed, after the priority date, this period being deemed to have been observed if the information is communicated before completion of the technical preparations for publication of the European patent application;
-
up to the date of submission of a request under Article 93, paragraph 1(b);
-
within one month after the European Patent Office has communicated to the applicant that the right to inspect the files under Article 128, paragraph 2, exists.
The ruling period shall be the one which is the first to expire. The communication of this information shall be considered as constituting the unreserved and irrevocable consent of the applicant to the deposited biological material being made available to the public in accordance with Rule 33."
Rule 33:
"(1) Biological material deposited in accordance with Rule 31 shall be available upon request to any person from the date of publication of the European patent application and to any person having the right to inspect the files under Article 128, paragraph 2, prior to that date. Subject to Rule 32, such availability shall be effected by the issue of a sample of the biological material to the person making the request (hereinafter referred to as “the requester”).
(2) Said issue shall be made only if the requester has undertaken vis-à-vis the applicant for or proprietor of the patent not to make the biological material or any biological material derived therefrom available to any third party and to use that material for experimental purposes only, until such time as the patent application is refused or withdrawn or deemed to be withdrawn, or before the European patent has expired in all the designated States, unless the applicant for or proprietor of the patent expressly waives such an undertaking.
The undertaking to use the biological material for experimental purposes only shall not apply in so far as the requester is using that material under a compulsory licence. The term “compulsory licence” shall be construed as including ex officio licences and the right to use patented inventions in the public interest."